Biosimilars are gradually becoming a growing part of the pharmaceutical market and are set to expand over the next decade. According to Netscribes research, by 2022, the global biosimilar market is expected to reach USD 36 billion, while the biologic market is expected to reach USD 261 billion. While biosimilars are already a multibillion-dollar business in the European market alone, it is relatively new in the US.
The approval of biosimilars by the US Food and Drug Administration has made way for the development of more biosimilars. Our recent study shows a strong pipeline of biosimilars that are in development. While many companies are interested in getting a share in the biosimilars industry, bringing these complex molecules into the market can be a challenge, not only during the development stage but also in terms of the manufacturing processes involved.
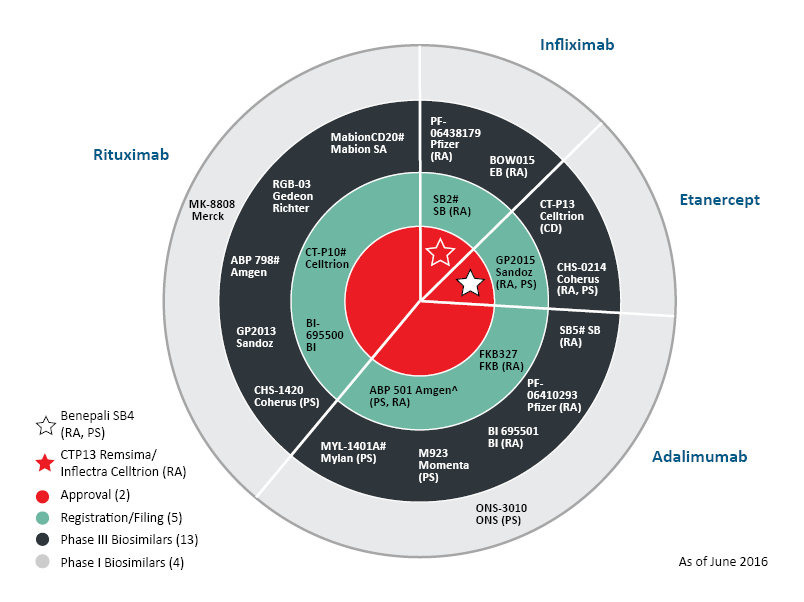
The US FDA has approved the second biosimilar (first monoclonal antibody) in the US, known as Inflectra (infliximab), which is similar to Janssen’s Remicade (infliximab). The infliximab by Pfizer and Celltrion is approved as a biosimilar and not as an interchangeable product. The same product was approved in Europe in 2013.
Netscribes estimates that around 16 to 20 biosimilars will be approved and launched between 2018 to 2021 in both US and EU.
Download our infographic Biosimilar Market Landscape Analysis for a closer look at the market favorability for biosimilars across developed, BRIC, and MIST countries, the number of biosimilars in the pipeline, top players in the market, and country-wise share of total biologic sales.
Speak to us for a detailed research on the current market landscape of biosimilars.